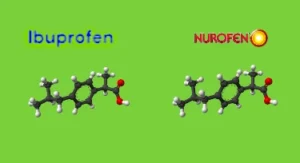
Generic medicines in the UK are identical to branded medicines in terms of their active ingredients, dosage, safety, strength, and intended use. Branded medicines are usually created by large pharmaceutical companies and can have a patent on their creation for up to 25 years. Once the patent has expired, other pharmaceutical companies can also create the same medicine but they have to keep to the same ‘recipe’ as the original manufacturer did. They are then approved by the Medicines and Healthcare products Regulatory Agency (MHRA), which ensures that these medicines meet strict standards of quality, efficacy, and safety. The MHRA does not grant approval for any generic medicine unless it is proven to be of the same high quality as its branded counterpart, guaranteeing that patients receive effective and reliable treatment.
